What is studied by the genealogical method in humans. Tasks and essence of the genealogical method for studying human heredity - stages and analysis
The concept of the genealogical method.
genealogical method, or pedigree analysis method, is the most fundamental and universal method for studying human heredity and variability. It consists in the study of some normal or more often pathological trait in generations of people who are related to each other. The genealogical method is based on genealogy - the study of genealogies. essence genealogical method is the compilation and analysis of pedigrees. The genealogical method corresponds to the main method of genetics - the hybridological method, which was first developed by G. Mendel. But unlike him, researchers do not select parental pairs for purposeful crossing, but only analyze in detail the results of the process of natural reproduction of people. One or several dozen families with numerous relatives of different generations are analyzed according to the trait under study. Usage a large number families partly compensates for low human fertility and increases the number of descendants studied.
Technically, the genealogical method includes two consecutive steps:
- Legend(verbal description of the pedigree). Collecting information about the members of the pedigree and compiling a verbal description of the pedigree indicating kinship and the presence or absence of the studied trait.
- Graphic representation of a pedigree. Drawing up a graphic image of a pedigree, its analysis and forecasting. Genealogy begins with proband- a person who has consulted a doctor. Most often, the proband is a patient or a carrier of the trait under study. When compiling pedigrees, special characters are used.
The graphic representation of a pedigree is a collection of symbols denoting males and females, some of which have a studied trait, while others do not have this trait. On the graphic image, all members of the pedigree are connected to each other by horizontal or vertical lines, reflecting family or marriage relations (husband - wife, parents - children). All individuals of one generation are located strictly in one row. Generations are indicated by Roman numerals from top to bottom; usually the numbers are placed to the left of the pedigree. All individuals of the same generation are numbered in Arabic numerals from left to right, sequentially. Siblings are listed in the pedigree in order of birth.
Recommendations for determining the type of trait inheritance.
To determine the type of inheritance of a trait, it is recommended to adhere to the following sequence of actions:
- Determine whether the trait under study is dominant or recessive.
If people with the studied trait are rarely found in the pedigree, not in every generation, and if the trait occurs in a person whose parents do not have the trait under study, then we can think that the studied trait is recessive.
If, on the contrary, people with the studied trait are often found in the pedigree, in every generation, and if children with this trait are born in those families where at least one of the parents has this trait, then we can think that the trait under study is dominant.
- Determine whether the gene that determines the formation of the studied trait is located in the autosome or on the sex chromosome.
If individuals of different sexes that have the trait under study occur with approximately the same frequency, for example, equally often or equally rarely, then we can think that the trait under study is autosomal, that is, the gene that determines it is located in the autosome.
If individuals of different sexes that have the studied trait occur with different frequencies up to the absence of the trait in representatives of the same sex, then we can think that the studied trait hooked to the floor: the gene that determines it is located on the sex chromosome. Analysis of the transmission of such a gene from generation to generation makes it possible to determine on which sex chromosome - X or Y - this gene is located.
- If the gene is located on the sex chromosome, then determine: on which sex chromosome - X or Y - is the gene that determines the formation of the trait under study. In this case, the following options are possible:
a) If the trait is found only in males and is transmitted only from father to son, then we can think that the gene under study is in Y-chromosome.
b) If in a particular pedigree a recessive trait is found only in males whose fathers do not have this trait, but their grandfathers or great-grandfathers on the maternal side have it, then we can think that the recessive allele that determines the development of the trait under study is located in X chromosome.
c) If among individuals with a dominant trait, females are approximately twice as common as males, and in a man with a dominant trait, all daughters also have this trait, and all his sons do not have this trait, then we can think that the dominant allele that determines the development of the studied trait is located in X chromosome.
- Having established the type of inheritance of the trait under study, check whether the analyzed pedigree has the traits that are characteristic of the type of inheritance you have chosen. Then make sure that the pedigree does not have a complex of traits that are characteristic of other types of inheritance.
As an example, we give a sequence of reasoning when determining the type of nuclear inheritance of a trait in the following pedigree
- People with the trait under study are found frequently, in every generation; a person with a studied trait is born in a family where at least one of the parents must have the studied trait. Therefore, we can make the first preliminary conclusion: the trait under study is dominant.
- In the pedigree, 6 women and 5 men have the studied trait. It can be assumed that the trait under study occurs with approximately equal frequency in both men and women. This is typical for traits whose genes are located not on the sex chromosomes, but on the autosomes. Therefore, we can draw a second preliminary conclusion: the trait under study is autosomal.
- Thus, according to the main features, the inheritance of the studied trait in this pedigree can be attributed to autosomal dominant type. In addition, this pedigree does not have a set of features that are characteristic of other types of inheritance.
However, the final conclusion can be made only by determining the genotypes of all members of the pedigree and making sure that only with an autosomal dominant type of inheritance is such a transfer of the gene that determines the development of the studied trait possible, and such a pattern of formation of the studied trait in them, which are reflected in the analyzed pedigree.
Despite its apparent simplicity, determining the type of inheritance in a particular pedigree real person is always a serious genetic problem, which can often be solved only by a geneticist.
Having determined the type of inheritance of the studied trait, one can easily find out the genotype of the proband and make a prediction about its probable offspring.
Determination of the proband genotype.
When determining the genotype of a proband, it is necessary to know the basic patterns of inheritance of genes and chromosomes and remember that a child can have in his genotype only those genes that his parents had and which he received from them during fertilization as part of germ cells.
Determining the genotype of the proband, we actually solve the simplest genetic problem for monohybrid crossing, where information about the traits of certain family members is presented not in the form of text, but in the form of a graphic image. For example, for the fragment of the genealogy shown in Fig. 4 and containing graphic images of the proband family members, such a text will look like this: “The trait under study is characterized by a recessive type of inheritance linked to the X chromosome. The boy has the trait being studied, while his parents, two older sisters and younger brother the trait being studied is missing. What is the boy's genotype? Therefore, when determining the proband genotype, it is necessary to adhere to the sequence of actions that is recommended when solving typical genetic problems.
In order to avoid errors in determining the proband genotype, we recommend determining not only the proband genotype, but also the genotypes of all members of the pedigree. In this case, it is recommended to follow the following sequence of actions.
- Next to the graphic representation of the pedigree, write the name of the alternative traits and the alleles that determine them, that is, draw a modified table “Target - gene”.
- Considering the pairing of chromosomes, the pairing of alleles of the autosomal gene under study and the peculiarities of the location of genes on the sex chromosomes, write down the future genotype using points to designate alleles. The preliminary record of the genotype will look like this:
- for autosomal inheritance: . . ;
- for X-linked inheritance: X . X . their . Y;
- for Y-linked inheritance: X X and X Y . .
- Those members of the pedigree who have the trait being studied, put the appropriate designation of the gene allele in place of the dot - a lowercase or capital letter:
- for autosomal and X-linked dominant inheritance: one allele A;
- for autosomal recessive inheritance: two alleles a;
- for recessive X-linked inheritance: X a X a and X a Y;
- for Y-linked inheritance, any image of a letter can be used.
- Put the corresponding designation of the allele of the gene instead of a dot to those members of the pedigree who have the studied trait absent:
- for autosomal dominant inheritance: two alleles a;
- for autosomal and X-linked recessive inheritance: one allele A;
- for dominant X-linked inheritance: X a X a and X a Y;
- for Y-linked inheritance, a letter image should be used that is different from that used to designate the genotype of an individual with the trait under study.
- Determine the alleles in those genotypes that cannot be determined from the individual's personal phenotype, but can be determined from the genotype of her parents or children. In this case, it must be taken into account that in the genotype of an individual there can be only those alleles that were in the genotype of its parents. For example, if, with an autosomal recessive type of inheritance, a woman does not have the trait being studied, but her father had this trait, then the genotype of this woman will contain the recessive allele A and will look like Ah. allele A we found by the phenotype of a woman, and the allele A- according to the genotype of her father. Indicate with an arrow the direction of transmission of the found allele from parent to child.
As an example, consider the sequence of reasoning when determining the genotype of a proband in a pedigree, a fragment of which has the following form:

The type of inheritance of the trait is recessive linked to the X chromosome.
To designate a gene that determines the development of the trait under study and its alternative, let's take one letter of the alphabet, for example, the letter "A". If the symbol “A” denotes the name of a gene, then the symbol A will denote the dominant allele of this gene, and the symbol a will denote the recessive allele of the gene “A”. Since, according to the condition of the problem, the gene under study is located on the X chromosome, the alleles of this gene must be recorded with the X chromosome.
Given the above, next to the fragment of the pedigree, we will write the names of alternative traits indicating the alleles of the “A” gene that determine them.
Next to the graphic image of each member of the pedigree, we will write its sex chromosomes, taking into account the sex of the person and putting a dot ( . ) at the place where the designation of the allele of the gene will be written later. After that, the fragment of the pedigree takes the following form.
Put the recessive allele A in the designation of the genotype of a member of the pedigree that has the trait under study. After that, the fragment of the pedigree takes the following form.

The mother of the proband has a recessive allele in her genotype A, since only she could pass on to the proband the recessive allele present on its X chromosome A(the proband received a Y-chromosome from his father). Draw an arrow from the genotype of the mother of the proband to the genotype of the proband, thus indicating the direction of transmission of the recessive allele A.
Sisters of the proband may have the genotype X A X A or X A X a, since the allele A they received from their father along with his X chromosome, and from their mother they can receive either an allele A, or allele
A with one or another of its X chromosomes. Let's write their possible genotypes under the genotypes of the proband's sisters, after which the fragment of the pedigree takes the following form.

Thus, we found that the genotype of the proband is X and Y, and made sure that we are really dealing with a pedigree that is characterized by a recessive type of inheritance linked to the X chromosome.
Calculation of the probability of a proband giving birth to a child with one or another alternative manifestation of a trait.
The most important stage of the genealogical method is the prediction of the proband's offspring. In the simplest cases, it consists in calculating the probability of a proband giving birth to a child with one or another alternative manifestation of the trait.
The probability of having a child with one or another alternative trait is defined as the proportion of children with this trait among the offspring with all the phenotypes that are only possible in the children of the analyzed parental pair. For example, with such an expected splitting (ratio) of offspring according to the phenotype, as 3 patients: 1 healthy, the probability of having a sick child will be ¾, and the probability of having healthy child – ¼.
It is recommended to calculate the probability of having a child with one or another sign in the following sequence:
- Write down the genotypes of the parents.
- Write the types of gametes that the parents produce.
- Write the splitting (ratio) of descendants F1 by genotype and phenotype.
- Calculate the probability of having a child with the phenotype (trait) you are interested in.
In the following example, get acquainted with the final record of calculating the probability of having a child from parents with known genotypes.
Example.
The task.
One of the universal and most commonly used methods in human genetics is genealogical.
genealogical method - compiling pedigrees and studying the inheritance of certain traits over a number of generations.
This method allows solving the following theoretical and applied problems:
There is a testable sign of hereditary (if relatives have it)
Type and nature of inheritance (dominant or recessive, autosomal or sex-linked)
Zygosity of individuals of the pedigree (hetero- or homozygous)
The frequency or likelihood of the phenotypic expression of a gene;
The probability of having a child with a hereditary pathology.
The genealogical method provides for the following stages of research: collection of data on all relatives of the subject, compilation of a pedigree, analysis of the pedigree and conclusions.
Collection of data on all relatives of the subject
A pedigree is usually made up of one or more traits. Depending on the purpose of the study, the pedigree can be complete or partial, but it is better to make the most complete pedigree in ascending, descending and lateral directions. The complexity of data collection lies in the fact that the examined carrier of the trait (proband) must know his relatives well and their health status on the maternal and paternal lines for at least three generations, which happens very rarely. However, polling is usually not enough. Some members of the pedigree have to appoint a full medical examination to clarify the state of their health.
drawing up a pedigree
To compile pedigrees, symbols are used (Fig. 3.1).
Rice. 3.1.
It is necessary to adhere to certain rules: the compilation of a pedigree begins with a proband, each generation is numbered on the left with Roman numerals, symbols denoting individuals of one generation are placed horizontally and numbered with Arabic numerals in the order of their birth. The basis of the pedigree is the proband, from which the genetic study of the family begins.
Pedigree analysis. First of all, the nature of the trait under study is determined. If this feature is manifested in a number of generations, then we can assume that it has a hereditary nature. After that, it is necessary to determine the type of trait inheritance. For this, genetic analysis techniques are used, as well as various statistical methods for processing data from many pedigrees.
Genetic analysis of pedigrees makes it possible to identify simple types of inheritance of traits - autosomal dominant, autosomal recessive and sex-linked.
Autosomal dominant inheritance pattern characterized by the fact that the gene of the trait under study is contained in a specific autosome and manifests itself both in the homozygous and in the heterozygous state. In the pedigree, it is determined by the following properties: the trait under study is present in each generation, regardless of gender, the manifestation of the trait is also observed horizontally - among brothers and sisters (Fig. 3.2).

Rice. 3.2. Genus with an autosomal dominant type of inheritance (brachydactyly, or short-fingered)
Depending on the zygosity of the parents for the alleles that control the trait, the birth of children with an autosomal dominant trait may have the following probability:
100% if at least one of the parents is homozygous for the dominant allele;
75% if both parents are heterozygous;
50% if one of the parents is heterozygous and the other is homozygous for the recessive allele.
Autosomal dominant traits are clearly manifested only under the condition of homozygosity. In heterozygotes, there is an intermediate phenotype for the trait under study. If this is a disease, then in the case of heterozygosity it may not manifest itself in every generation.
By autosomal recessive inheritance pattern the gene of the studied trait is located in the autosome, and shows its effect only in the homozygous state. This type of inheritance is characterized by the following features: the studied trait is not present in every generation, a child with a trait can be born to parents who do not have it (heterozygous parents), the trait occurs with the same frequency regardless of gender and is observed horizontally (Figure 3.3).

Rice. 3.3. Genus with autosomal recessive inheritance (albinism)
The probability of inheriting an autosomal recessive trait, depending on the zygosity of the parents for the alleles that control the trait, can be as follows:
25% if both parents are heterozygous;
50% if one of the parents is heterozygous and the other is homozygous for this recessive gene;
100% if both parents are homozygous for the recessive allele.
In the case of a hereditary disease of an autosomal recessive type, the probability of inheritance is 25%. Such patients either do not survive to the onset of puberty, or do not marry.
sex-linked inheritance can be X-linked dominant, X-linked recessive and B-linked. This means that the gene that controls the trait under study is contained in the sex chromosomes - X or Y.
1. X-linked dominant type of inheritance. It has the following properties: women with this trait are twice as many as men; the trait manifests itself in every generation; the father-bearer of the sign passes it on to all daughters, but does not pass it on to his sons; the mother-carrier of the trait can pass it on to half of her children, regardless of gender; in children, the sign will appear when at least one of the parents carries it; children of parents deprived of signs are also without it. An example of such a sign can be the brown color of tooth enamel (Fig. 3.4).

Rice. 3.4. Rodovid with X-linked dominant type of inheritance (brown color of tooth enamel)
2. X-linked recessive type of inheritance. It is characterized by the following properties: the trait is not present in every generation; a child with a sign can be born to parents deprived of it, the sign manifests itself mainly in men and, as a rule, horizontally; a father deprived of a trait is not a carrier of the allele for that trait and does not pass it on to his daughters.
If a woman without a sign marries and a man with a sign, then all their children will be without a sign. Daughters will receive an X chromosome with a trait gene (recessive) from their father and will be heterozygous carriers, so they will receive a second X chromosome (with a dominant gene) from their mother.
In a man without a sign and a woman with an allele of signs, the probability of having a boy with a sign is 50% of all children and 25% of all children.
The chance of girls being born with the trait is very low, and this is possible only when the father has the trait, and have a heterozygous carrier of the gene for the trait. In this case, half of the girls will be with the trait, and the second half will carry the allele in the heterozygous state.
A classic example of inheritance of X-linked recessive traits can be hemophilia, which causes increased bleeding due to a lack of blood clotting factors in the body (Fig. 3.5).

Rice. 3.5. Rodovid with X-linked recessive type of inheritance (hemophilia)
3. B-linked inheritance or hollandric. It is characteristic only male gender. The human Y chromosome contains very few genes that are passed down from father only to sons. At the same time, the trait is present in all generations and in all men. An example of hollandric inheritance can be the inheritance of hypertrichosis (the presence of hair along the edge of the auricles (Fig. 3.6).

Rice. 3.6. Rodovid with Y-linked type of inheritance (hypertrichosis)
The genetic method can also be used in diagnosing diseases with a hereditary predisposition, the inheritance of which is subject to Mendel's law.
A person as an object of genetic research has almost no advantages over other objects.
On the contrary, there are many obstacles that make it difficult to study its genetics: 1) the impossibility of arbitrary crossing in the experiment; 2) late onset of puberty; 3) a small number of descendants in each family; 4) the inability to equalize living conditions for offspring; 5) the lack of accurate registration of the manifestation of hereditary properties in families and the absence of homozygous lines; 6) a large number of chromosomes; 7) and the most important difficulty in studying human genetics in a capitalist society is social inequality, which makes it difficult to realize a person's hereditary potentials.
Despite these difficulties, genetics has developed some methods that allow you to study heredity and inheritance in humans step by step. There are several research methods: genealogical, cytogenetic, twin, ontogenetic and population.
It should be borne in mind that any trait, regardless of whether it is a wild type trait, i.e., refers to the norm, or is associated with any disease, can serve as a model for the study of heredity. Protecting a person from hereditary diseases or damage to his heredity is as important as finding out the inheritance of the norm. At present, genetic methods have been developed mainly in relation to morphological traits that are genetically determined quite clearly (brachydactyly, albinism, color blindness, spotting of the skin and hair, etc.).
The genetic study of mental properties still remains problematic, since elementary criteria for a trait in the genetic sense have not been found for them. Almost all signs of a person’s mental and creative activity are so complex and complex, and are also largely determined by external, including social, factors that it is still difficult to carry out a genetic analysis of these properties, although their hereditary conditionality is beyond doubt.
It can be said that the great majority of the traits that characterize the species Homo sapiens can be studied as quantitative and complex physiological traits, i.e., traits that do not show a discrete character in ontogeny. These traits are controlled by the genotype system (polygenic). And until this system is unraveled, at least on the example of simply organized organisms, the problem of signs of behavior remains inaccessible for genetic analysis. On the contrary, mutant traits that go beyond the characteristics of species traits serve as good genetic models for studying heredity and inheritance in the norm.
Discrete mutant traits cannot be viewed as only pathological traits, allegedly having no adaptive value. It is possible that the very appearance of a person with developed hemispheres of the cerebral cortex, a vertical position of the body, and discrete speech signaling is the result of major mutations. This is strongly supported by
a short period of time in human evolution, during which small mutations could hardly accumulate in such a quantity and give such a significant evolutionary effect. A reasonable person is as “unusual” for nature as a domestic chicken that lays 365 eggs a year instead of 10-15, or a record-breaking cow that gives 16 thousand kg of milk a year instead of 600-700 kg.
The division of traits into normal and mutant in relation to humans and animals is necessary for the knowledge of human evolution and pathological phenomena.
The totality of species characteristics of humans and animals is determined by the genotype system, which has developed under the influence of all selection factors in the process of evolution. Mutations that are heterozygous in humans seem to be just as necessary as in animals to maintain them in the population.
The most dangerous thing in the development of scientific methods for the study of animals and man, especially his abilities, is the anthropomorphic moment, that is, wishful thinking.
genealogical method
An analysis of human inheritance based on the compilation of a pedigree - genealogy was proposed by F. Galton.
genealogical method is a study of the inheritance of human properties according to pedigrees (pedigry). This method is applicable if direct relatives are known - the ancestors of the owner of the hereditary trait (proband) on the maternal and paternal lines in a number of generations and there are a sufficient number of descendants in each generation, or in the case when there are data on a sufficient number of different families to reveal similarities pedigrees. Data on a set of similar pedigrees is subjected to statistical processing.
The most widely used system for designating human pedigrees was proposed by G. Yust in 1931.
On the basis of a large number of analyzed families, pedigrees are compiled and mathematical calculations are made according to the type of inheritance of a particular trait - dominant or recessive, often and not often occurring mutations, sex-linked or not, etc. Here we will not touch on the application of the mathematical method to this analysis, we only note that this entire formal analysis is based on elementary genetic patterns of inheritance.
Pedigree inheritance patterns of a dominant autosomal gene that determines a trait, such as a disease (chondrodystrophic dwarfism, bullous epidermolysis - the ability of the skin to form large blisters with minor injuries, retinoblastoma, etc.), or a morphological defect, such as short fingers (brachydactyly - the absence of two distal phalanges in fingers).

The inheritance of traits determined by recessive genes (recessive inheritance) is analyzed somewhat more complicated when drawing up pedigree charts.
For example, two in a family, the appearance of two sick children is equal to the product of probabilities, i.e. 0.25 X 0.25, i.e. 6.25%.
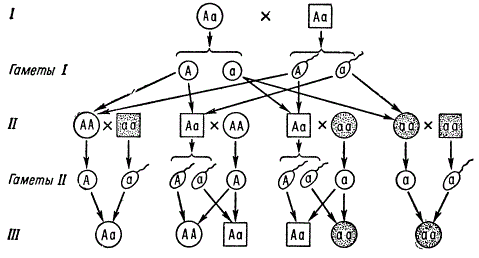
Frequently occurring recessive autosomal genes, provided that their carriers (aa) are able to marry and produce offspring, will be in high concentration in the population. In this case, marriages aa X Aa become very likely, in the offspring from which the inheritance of this trait will imitate inheritance according to the dominant type 1: 1. However, knowing the type of inheritance and manifestation of those and other genes, even in the case of small families, but with a sufficient number of such families, it is possible to establish the true nature of inheritance.
The inheritance of genes that are fully sex-linked, i.e., located in non-homologous segments, and partially sex-linked - localized in homologous segments of X- and Y-chromosomes, obeys the patterns established for sex chromosomes. For dominant and recessive genes, this inheritance will be defined differently depending on where the gene is located - in the homologous or non-homologous segment of the X and Y chromosomes, and how it is transmitted. So, the dominant gene that causes webbed fingers, located in the non-homologous segment of the Y chromosome, is inherited from fathers and manifests itself only in men.
For partially sex-linked dominant genes located in homologous segments of the sex chromosomes, analysis is somewhat more difficult, but also possible. An example of sex-linked inheritance of a recessive trait is the inheritance of hemophilia. There is a discontinuity in the transmission of this trait in generations; affected males are descendants of healthy mothers who were heterozygous for the gene; women with hemophilia can be descendants of a sick father and a sick or healthy mother.
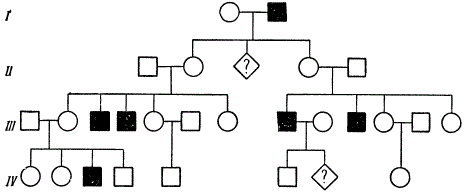
About 50 sex-linked recessive genes have been found in humans. Interestingly, about half of them cause eye disease. It has long been known that the degree of transmission of hereditary traits in related (inbreeding) and unrelated marriages (outbreeding) is different. Once genetics has established patterns of more frequent manifestation of recessive genes during inbreeding, there is no need to argue at length about the harm of consanguineous marriages. The higher the inbreeding coefficient, the more likely the occurrence of hereditary diseases in generations. IN different countries among different peoples and classes of society, as well as in different eras related marriages (between cousins, second cousins and sisters) occur with different frequency. So, for example, in villages on the Fiji Islands, the number of related marriages reaches 29.7%, in some castes of India - 12.9, in Japan (Nagasaki) - 5.03, in Holland - 0.13-0.159, in Portugal - 1 40, in the USA (Baltimore) - 0.05%, etc. The percentage of consanguineous marriages varies in different regions of the same country, depending on the way of life.
The harmfulness of consanguineous marriages is hardly noticeable in individual pedigrees, but with a comparative statistical analysis of diseases and mortality, it appears with complete obviousness.
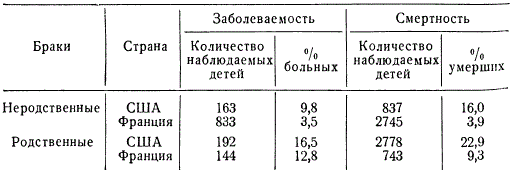
A striking example of the detection of a recessive gene in consanguineous marriage.
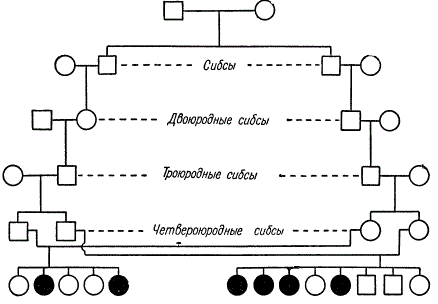
In this pedigree, kinship is maintained through the marriage of siblings (brothers and sisters) of varying degrees of kinship. From two consanguineous marriages (fourth cousin siblings) 4 out of 8 children appeared in one family, and 2 out of 5 in the other, suffering from hereditary amaurotic idiocy. K. Stern suggests that one of the two common ancestors of these lines passed this recessive gene through three generations to each of the four parents.
Sometimes the disease and mortality of children from related marriages exceed by 20-30% those from unrelated marriages. Obviously, the reason for the phenomenon under consideration is genetic, namely: a high probability of the manifestation of hereditary diseases and mortality as a result of homozygotization of recessive genes that determine physiological deficiencies and mortality (lethal and semi-lethal genes).
So, the genealogical method is a very valuable method, but its importance in research is the greater, the more accurate and deeper the pedigrees are compiled. With the growth of civilization and more accurate registration of pedigrees, the role of this method in human genetics will increase.
twin method
twins called the offspring, consisting of simultaneously born individuals in singletons (humans, horses, cattle, sheep, etc.).
Twins can be identical or fraternal.
identical, or identical, twins(OB) develop from one egg fertilized by one spermatozoon, when two or more embryos arise from a zygote instead of one embryo (polyembryony). Due to the fact that the mitotic division of the zygote gives two equally hereditary blastomeres, identical twins, no matter how many they develop, must be hereditarily identical and of the same sex. This phenomenon is an example of asexual, or rather, vegetative reproduction of animals.
fraternal twins(RB) develop from simultaneously ovulated different eggs, fertilized by different spermatozoa. And since different eggs and sperm can carry different combinations of genes, fraternal twins can be as genetically different as children of the same married couple who were born at different times. Fraternal twins can be of the same (RBR) or different sex (RBR).
More often in the literature, instead of the term "fraternal twins" (RB), the term "fraternal twins" (DB) is used, since twins are more common. However, the term "fraternal twins" better emphasizes the difference between OB and RB; identical twins are also more likely to be born as twins.
Judging by the data obtained on mammals, there can be several hypotheses to explain the formation of OB in humans:
- divergence of blastomeres during the first division of the zygote and separate development of the embryo from these blastomeres;
- separation of a group of cells at the blastocyst stage (before gastrulation);
- separation of embryos at an early stage of gastrulation. The most likely way is the second one.
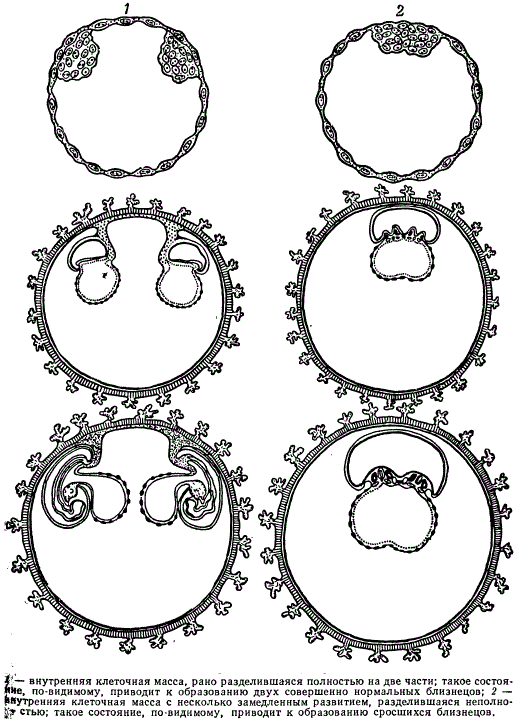
The number of twins in one birth in a person varies: twins are most common, triplets are less common, quadruples are even rarer, and five are very rare. According to I. I. Kanaev, over the past 150 years, four cases of quintuplets have been found in the United States, and two cases in Canada. The fact of the birth of OB - five girls who survived to adulthood - is known in the family of the Canadian farmer Dionne (1934). It is calculated that quintuplets are born once in 54,700,816 births, gears - in 4,712 million births, septuplets are known only as an exception. On average, the birth rate of twins is 1% with fluctuations in the range of 0.5-1.5%. Twins are less viable, and therefore their number at birth is less than at conception, and in adulthood less than at birth.
The calculation of the frequency of OB in relation to RB is based on the theoretical ratio of same-sex and opposite-sex couples of RB at the birth of twins: 25%♀♀ + 50%♀♂ + 25%♂♂ female) will give a difference in the number of OB pairs, which on average ranges from 21 to 33.4% of all twins.
For the use of twins in genetic studies, it is very important to accurately diagnose the type of AB and the type of RP. Diagnosis is made on the basis of the following criteria:
- OBs must be of the same sex, RBs can be of the same sex or different sexes;
- OB have, as a rule, one common chorion, RB - different chorions;
- reciprocal tissue transplantation in OB is as successful as autotransplantation, it is impossible in RB;
- the presence of similarity (concordance) in OB and dissimilarity (discordance) in RB in many ways.
For diagnosis, one should choose signs that are clearly inherited and are least susceptible to change under the influence of environmental factors; such signs include blood groups, pigmentation of the eyes, skin and hair, skin relief (fingerprints, palms, feet, etc.). If one or two of these features reveal a difference between twins, then they are, as a rule, RB.
All dubious cases of twin diagnosis can be caused either by a developmental disorder of one of the OB partners, or by the similarity of the parents in a number of ways. However, the latter is extremely rare. It should be noted that developmental disorders of one of the OB partners are usually explained by the unequal effects of prenatal life factors and the occurrence of somatic mutations in the early stages of embryonic development, before organ formation. Various gene and chromosomal rearrangements, monosomy and other mutations that occur in one of the partners can cause significant differences in the OB phenotype. Therefore, it is necessary to take into account the possibility of somatic mutations in OB in early embryogenesis.
According to the generalizations of I. I. Kanaev, outlined in his excellent monograph, the essence of the twin method in genetics is reduced to the following provisions:
1) the OB pair has an identical combination, the RB pair has different combinations of the genotypes of the parents;
2) for both partners of one pair of OBs, the external environment may turn out to be the same, and for the other - different. If OB partners experience different influences throughout their lives, this will lead to an intra-pair difference. Hence, pairs can be with intra-pair identical and intra-pair different environments.
Comparison of OBs with the same environment with OBs with a different environment opens up the possibility of judging the role of the influence of the environment on intra-pair differences in twins throughout life. Comparison of OB with the same environment and RB with the same environment allows you to find out the role of the hereditary factor. This kind of study is carried out on a large sample and processed statistically.
Based on the difference in the genetic origin of OB and RB, it follows that if there are no differences in OB for the same traits and there are such in RB, then it is obvious that these differences in traits in the latter are due to hereditary factors. If, however, intra-pair differences in the same traits occur in one and the other type of twins, then it is obvious that they can be caused by environmental factors. From the data of discordance in OB and RB for a number of morphological features, it can be seen that the intra-pair difference in RB occurs many times more often than in OB.

Some data of S. Reed on the comparative frequency of pathology in the second partner in the case of illness of one of the twins are presented.

The percentage shows the frequency of disease concordance in two types of twins, it shows that if one partner fell ill with one of the indicated diseases, then the probability of the second disease in OB is much higher than in RB. VP Efroimson, analyzing the data on the frequency of contortional pairs, quite correctly indicates that a high hereditary predisposition of OB to diseases manifests itself in the presence of a provoking factor; without it, this percentage will be much lower.
The twin method makes it possible to determine with the greatest accuracy the hereditary predisposition of a person to a number of diseases and properties. By other methods it is very difficult or almost impossible to study many infectious and tumor diseases, inflammations of the skin and various organs, as well as the characteristics of normal human nervous activity.
When using the twin method, one has to take into account the conditions of joint and separate upbringing in the lives of partners, the social conditions in which they are located, etc. the studied genes and isolate the role of the environment in determining the variability of the studied traits.
Cytogenetic method
Cytogenetic method in human genetics, a cytological analysis of a person's karyotype in normal and pathological conditions is usually called.
It is more correct to call this method cytological rather than cytogenetic, since genetic analysis by crossing in humans is excluded, and carriers of chromosomal disorders, if they survive, are usually infertile. However, occasionally, in relation to some chromosomal disorders, it is possible to combine the cytological method with the genealogical one and to establish a connection between the phenotypic effect and a certain type of chromosomal changes. Due to these circumstances, it is possible to retain the term "cytogenetic method" accepted in the literature in the study of human genetics. In those cases where such parallelism is not being studied, the use of this term is not authorized.
The cytogenetic method is used to investigate various kinds of heteroploidy and chromosomal rearrangements in human somatic tissues, causing various phenotypic deviations from the norm.
Most often, this method is used in tissue culture. It allows to take into account large chromosome anomalies that occur both in germ and somatic cells. It turned out that in humans, as well as in animals, trisomics and monosomics quite often arise for different pairs of chromosomes due to non-disjunction of autosomes and sex chromosomes during meiosis. Trisomy and monosomy for sex chromosomes in humans are detected based on the analysis of sex chromatin.
In the course of a relatively long individual development of a person, chromosome anomalies (chromosomal rearrangements, as well as a change in the number of chromosomes) accumulate in the cells of various tissues. Body tissues are diverse populations of genetically different cells, in which the concentration of cells with pathological nuclei increases with age. In this case, the cytogenetic method makes it possible to study tissue aging based on the study of cell structures in the age dynamics of the "population" of somatic and generative tissues.
Since the frequency of occurrence of chromosomal abnormalities depends on the influence of various mutagens on the body (ionization, chemical agents - pharmacological preparations, the gas composition of the environment, etc.), the cytogenetic method makes it possible to establish the mutagenic effect of factors external environment per person.
The use of the cytogenetic method has especially expanded in connection with the discovery of the causes of a number of physical and mental diseases - the so-called chromosomal diseases.
There are several human diseases, for example, Klinefelter's disease, Shereshevsky-Turner's disease, Down's disease, and others, the causes of which remained unknown for a long time until chromosomal abnormalities were detected in such patients by cytological methods.
Sick men with Klinefelter's syndrome are characterized by underdevelopment of the gonads, degeneration of the seminiferous tubules, mental retardation, disproportionate growth of the limbs, etc. Shereshevsky-Turner syndrome occurs in women. It manifests itself in delayed puberty, underdevelopment of the gonads, absence of menstruation, infertility, short stature, and other pathological signs.
It turned out that both of these syndromes in offspring are the result of nondisjunction of sex chromosomes during the formation of parental gametes. Due to the non-disjunction of the X chromosomes in the female homogametic) sex, during meiosis, gametes can occur with two X chromosomes, i.e. XX + 22 autosomes, and without X chromosomes, i.e. 0 + 22; in the male (heterogametic) sex, respectively, the gametes XY + 22 and 0 + 22. In the case of fertilization of such eggs by normal spermatozoa (X + 22 or Y + 22), the following classes of zygotes can be formed: XXX + 44, 0X + 44 and XXY + 44, 0Y+44.
It follows from this that the number of chromosomes in zygotes of different origin can vary from 47 to 45, and individuals 0Y + 44, obviously, do not survive, since they have never been found. The chromosome set XXY + 44 is inherent in a man with Klinefelter's syndrome (male intersexuality), the chromosome sets X0 + 44 and XXX + 44 are found in women with Shereshevsky-Turner syndrome.
Upon further analysis of patients with different syndromes, it turned out that due to nondisjunction of sex chromosomes, different type chromosomal abnormalities, in particular polysomy. There are, for example, men with such sets of chromosomes: XX Y, XXX Y, XXXX Y, and women - XXX, XXXX.
The peculiarity of the role of sex chromosomes in the determination of sex in humans in the case of their non-disjunction, in contrast to Drosophila, was manifested in the fact that the XX Y chromosome set always determines the male sex, and the X0 set determines the female sex. At the same time, an increase in the number of X chromosomes in combination with one Y chromosome does not change the definition of male, but only enhances Klinefelter's syndrome. Trisomy, or polysomy on the X chromosome, in women also often causes diseases similar to Shereshevsky-Turner syndrome.
Diseases caused by a violation of the normal number of sex chromosomes are diagnosed by a cytological method - analysis of sex chromatin. In those cases when the tissues of men have a normal set of sex chromosomes - XY, sex chromatin is not found in the cells. In normal women - XX - it is found in the form of a single body. With X chromosome polysomy in women and men, the number of sex chromatin bodies is always one less than the number of X chromosomes, i.e. n x \u003d n X - 1. So, in the cells of men with Klinefelter syndrome, when XX Y is recruited, there is one body sex chromatin, with a set of XXXY - two, with a set of XXXXY - three; in women with Shereshevsky-Turner syndrome, respectively: X0 - no body, XXX - two bodies, XXXX - three sex chromatin bodies, etc. It is assumed that only one of the X chromosomes is genetically active in each such zygote. The rest of the chromosomes go into a heteropyknotic state in the form of sex chromatin.
The reasons for this pattern have not been elucidated, but it is assumed that it is associated with the leveling of the action of sex chromosome genes in the hetero- and homogametic sex.
As we know, nondisjunction of chromosomes can occur not only in meiosis, but also in somatic cells during the entire embryogenesis of an animal, starting from the first cleavages of the egg. Due to the latter, among people with a violation of the divergence of the sex chromosomes, sick female mosaics and male mosaics may appear. For example, mosaics of the following types are described: double: X0/XX, X0/XXX and X0/XY, X0/XYY, triple: X0/XX/XXX, XX/X0/XY, as well as quadruple mosaics, when somatic cells of one people contain four different set chromosomes.
In addition to the considered type of diseases caused by a change in the number of sex chromosomes in the zygote, chromosomal diseases can be caused by nondisjunction of autosomes and various kinds of chromosomal rearrangements (translocations, deletions). So, for example, in children with congenital idiocy - Down's disease, accompanied by short stature, wide round face, closely spaced by narrow palpebral fissures and a half-open mouth, trisomy on chromosome 21 was detected. It has been established that the incidence of Down's disease in newborns depends on the age of mothers.
A wide variety of diseases are associated with congenital chromosomal abnormalities. Therefore, the cytogenetic method is of great importance in the etiology of human diseases.
population method
population method allows you to study the distribution of individual genes or chromosomal abnormalities in human populations.
The population method is based on mathematical methods. To analyze the genetic structure of a population, it is necessary to examine a large sample, which must be representative - objectively reflect the entire general population, i.e., the entire population as a whole. In the examined sample, the distribution of persons is established according to the corresponding clearly defined phenotypic classes, the differences between which are hereditarily determined. Then, based on the found phenotypic frequencies, gene frequencies are determined.
Based on the knowledge of gene frequencies, it is possible to describe the analyzed population in accordance with the Hardy-Weinberg formula and predict in advance the likely nature of splitting in the offspring of individuals belonging to one or another phenotypic class. The study of gene frequencies is important for assessing the consequences of consanguineous marriages, as well as for elucidating the genetic history of the human population as a whole.
The frequency of distribution in populations of different anomalies turns out to be different; while the vast majority of the corresponding recessive alleles are presented in the heterozygous state.
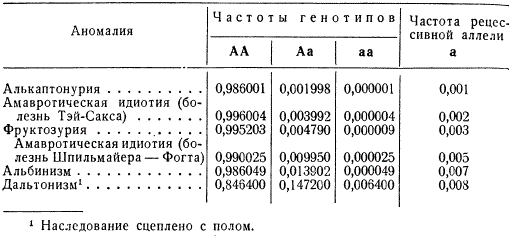
Thus, approximately every hundredth inhabitant of Europe is heterozygous for the gene of amaurotic idiocy (Spielmeier-Vogt disease), while only 25 people out of 1 million who are homozygous fall ill with this disease in adolescence. Albinos in European countries occur with a frequency of 1 in 20,000, although the heterozygous state of this allele is inherent in every seventieth inhabitant.
The situation is somewhat different in the case of anomalies that are inherited sex-linked, an example of which is color blindness - color blindness, which is controlled, apparently, by a number of alleles distributed over two closely linked loci on the X chromosome. Among the male population, the frequency of color blindness (q) corresponds to the total frequency of recessive alleles and was, for example, in Moscow in the 30s, according to R. I. Serebrovskaya, 7%, while at the same time, among the female population of the same population, color blindness was only 0.5% (q 2), but in the heterozygous state, approximately 13% of women carry alleles that cause color blindness.
As we said above, considering the genealogical method, the probability of the appearance of recessive homozygotes in the offspring may be different when persons with different degrees of kinship marry. So, for spouses who are in relation to each other cousins and sisters, the probability of having children homozygous for the recessive allele, common in the population with a frequency q, will no longer be q 2, but a large value, namely q / 16 (1 + 15q).
This is due to the fact that if one of the common ancestors of such spouses - a grandmother or grandfather - carried a recessive allele in the heterozygote, then with a probability of 1/16 this allele will be transmitted to both cousins.
The harmful effects of consanguineous marriages are particularly evident in isolated populations of limited size, the so-called isolates. An isolate is a group of individuals of a population that mostly marry individuals of their group and therefore are characterized by a significant coefficient of consanguinity. Such isolates can be separate isolated villages, communities, etc. Within an isolate, related marriages (inbreeding) are more likely, and spouses are more likely to carry the same mutant genes, which results in an increase in the likelihood of manifestation of recessive alleles in the homozygous state. Different isolates carry different concentrations of similar or different genes.
In the Marianas and the island of Guam, the mortality among the local population from amyotrophic lateral sclerosis (associated with damage to the cells of the anterior horns of the spinal cord) is more than 100 times higher than the mortality from this disease in other countries. In southern Panama, in the province of San Blas, a very prominent part of the Carib Kuna tribe are albinos, who appear here in every generation. In one village on the river. Rhone in Switzerland, among 2,200 inhabitants, there are more than 50 deaf-mutes, and 200 more have some hearing impairments. In all likelihood, in all such cases of a sharp increase in the concentration of individual alleles, a certain role is played by genetic drift, uneven reproduction in the past of individual families, genera, and a decrease in migration.
As civilization grows and the productive forces of society develop, the number of isolates decreases, and their importance for the population as a whole decreases. However, they still exist.
Knowledge of gene frequencies, as already mentioned, makes it possible to predict the nature of splitting in the offspring of individual phenotypic classes of parental individuals.
Based on the Hardy-Weinberg formula, it can be shown that with monogenic inheritance, splitting by phenotype in the offspring of dominant mothers should be carried out in the ratio p (1 + pq) of dominants to p recessives, or (l + pq): q 2; in the offspring of recessive mothers, the phenotype split should be pq 2: q 3 , or p: q.
Let's take an example. In one study, when studying the Rh factor, the frequency of the recessive rh allele in the population was 0.4, and the frequency of the dominant allele of Rh was 0.6. Hence, it was to be expected that in the offspring of Rh-positive mothers, the frequency of Rh-positive children (Rh +) would be approximately 7.8 times higher than the frequency of Rh-negative children (Rh -); in the offspring of Rh-negative mothers, the corresponding excess will be 1.5 times.
The actual ratios in the surveyed sample were:
- in the first case 1475 Rh + : 182 Rh - , or 8.1: 1,
- in the second case 204 Rh + : 129 Rh - , or 1.6: 1.
Thus, the observed splitting results are in very good agreement with the theoretically expected results predicted from gene frequency analysis.
Population analysis of polymorphism by blood groups is interesting in that it helps to understand the dynamics of the genetic structure of different populations and helps to identify relationships between them.
Different populations differ significantly in their genetic structure, in particular in blood types. At the same time, it is possible to trace some quite clear patterns. If the concentration of the I B allele is highest in the region of India and China, then to the east and west of this region it gradually drops down to zero among the indigenous inhabitants of America and Australia. At the same time, in the American Indians (and the natives of Australia and Polynesia), the concentration of the allele I 0 reaches a maximum. Allele I A is rare in the indigenous population of America, as well as in India, Arabia, tropical Africa, and Western Europe.
To explain these differences in the genetic structure of populations, a hypothesis has recently been proposed, according to which the decisive factor in the selection in relation to the blood groups of the AB0 system was plague and smallpox epidemics. The causative agent of the plague, Pasteuvella pestis, having the property of antigen 0, is most detrimental to people with blood type 0, since such individuals are not able to produce enough antibodies in the event of an infection. For a similar reason, the smallpox virus is most dangerous for people with blood group A. Where the plague raged (India, Mongolia, China, Egypt), there was an intensive elimination of the I 0 allele, and where smallpox was especially rampant (America, India, Arabia, tropical Africa), the allele 1 A was eliminated first of all. In areas of Asia, where plague and smallpox were endemic, the allele 1 c received the highest frequency.
In Chapter 5, we reviewed the monogenic inheritance of sickle cell anemia due to splitting of the S alleles. the result is a system of balanced hereditary polymorphism.
Thus, in both examples of the analysis of polymorphism by blood groups and sickle cell anemia, we see how the use of the population method allows us to reveal the genetic structure of human populations.
ontogenetic method
ontogenetic method allows you to establish the carriage of recessive alleles in the heterozygous state and chromosomal rearrangements by phenotype.
The genetic basis for the manifestation of recessive genes in the heterozygous state is, apparently, an incomplete block in the synthesis chain of one or another metabolite caused by the action of the dominant allele of this gene.
It is known that some hereditary diseases manifest themselves not only in persons homozygous for the alleles that cause the disease, but in an erased form in heterozygotes as well. Therefore, methods for determining heterozygous carriage in ontogeny are currently being intensively developed. So, a heterozygous carrier of phenylketonuria (an increased content of phenylalanine in the blood is determined by the additional administration of phenylalanine and subsequent determination of its (or tyrosine) level in the blood plasma. The presence of heterozygosity for this allele is established by an increased content of phenylalanine. alleles) the level of phenylalanine does not change. Normally, the catalase enzyme necessary for carbohydrate metabolism is present in the blood, but there is a gene that causes the absence of catalase in the homozygous state. catalase activity without much overlap between dominant and recessive homozygotes.
Catalase activity can accurately identify heterozygous and homozygous carriers of the acatalase allele among close relatives and parents.
Heterozygous carriage of the allele that determines Duchenne muscular dystrophy is tested by the activity of creatine phosphokinase. Now similar tests have been developed for 40 hereditary diseases determined by recessive alleles.
At present, the ontogenetic method has been enriched by biochemical, immunological, and molecular methods of research, which are described in a number of special manuals.
The importance of the ontogenetic method is obvious for establishing the carrier state of a recessive gene in a heterozygous state in relatives of a family in which a hereditarily ill child appears. Diagnostics in ontogenesis is important for calculating the probability of the appearance of hereditarily diseased offspring with related and mixed marriages. As heterozygous carrier testing becomes easier, this method will need to be introduced to counsel couples about the possibility of their children developing the disease, as well as to study the spread of mutations in populations.
If you find an error, please highlight a piece of text and click Ctrl+Enter.
The basic patterns of heredity established for living organisms are universal and fully valid for humans. However, as an object of genetic research, a person has its advantages and disadvantages.
It is impossible for humans to plan artificial marriages. Back in 1923, N.K. Koltsov noted that "... we cannot experiment, we cannot force Nezhdanova to marry Chaliapin just to see what kind of children they will have." However, this difficulty can be overcome thanks to the targeted selection of a large number of marriage pairs of those that correspond to the goals of this genetic study.
A large number of chromosomes - 2n = 4b - greatly complicates the possibilities of human genetic analysis. However, the development of the latest methods of working with DNA, the method of hybridization of somatic cells and some other methods eliminate this difficulty.
Due to the small number of descendants (in the second half of the 20th century, 2-3 children were born in most families), it is impossible to analyze splitting in the offspring of one family. However, in large populations, families with traits of interest to the researcher can be selected. In addition, in some families, certain signs have been traced over many generations. In such cases, genetic analysis is possible. Another difficulty is related to the duration of generational change in humans. One generation in humans takes an average of 30 years. And, therefore, a geneticist cannot observe more than one or two generations.
A person is characterized by a large genotypic and phenotypic polymorphism. The manifestation of many signs and diseases is highly dependent on environmental conditions. It should be noted that the concept of "environment" for humans is broader than for plants and animals. Along with nutrition, climate, and other abiotic and biotic factors, human environment is also social factors that are difficult to change at the request of the researcher. At the same time, a person as a genetic object is widely studied by doctors of all specialties, which often helps to establish various hereditary abnormalities.
Currently, interest and attention to the study of human genetics is actively increasing. Yes, global international program The "Human Genome" aims to study the human genome at the molecular level. To solve it, all the latest modern methods of genetics and medicine are used.
What methods does human genetics have today? There are many of them: genealogical, twin, cytogenetic, population-statistical, biochemical, somatic cell genetics and molecular genetics. Let's take a closer look at each of them.
Being one of the main ones in human genetics, this method is based on genealogy - the doctrine of genealogies. Its essence is the compilation of a pedigree and its subsequent analysis. This approach was first proposed by the English scientist F. Galton in 1865.
genealogical method is widely used to solve both scientific and applied problems. It allows you to identify the hereditary nature of the trait and determine the type of inheritance. Along with this, the method makes it possible to establish linked inheritance, determine the type of gene interaction and allele penetrance. The genealogical method underlies medical genetic counseling. It includes two stages: compilation of pedigrees and their genealogical analysis.
Drawing up a pedigree. The collection of information about the family begins with a person called a proband. This is usually the patient with the disease being studied. Children of one parental couple are called sibs (brothers and sisters). In most cases, a pedigree is collected on one or more grounds. Pedigree can be full or limited. The more generations traced in the pedigree, the more complete it is and the higher the chances of obtaining completely reliable information. The collection of genetic information is carried out by interviewing, questioning, personal examination of the family. The survey usually begins with relatives on the maternal side: maternal grandparents, indicating the grandchildren, children of each child of the grandparents. The pedigree includes information about miscarriages, abortions, stillborns, infertile marriages, etc.
When compiling a pedigree, a brief record of data on each member of the genus is kept, indicating its relationship in relation to the proband. Usually, the following are indicated: surname, name and patronymic, date of birth and death, age, nationality, place of residence of the family, profession, the presence of chronic diseases in the family, the cause of death of the deceased, etc.
After collecting information, they make a graphic representation of the pedigree using a system of symbols (Fig. 2.1).
In doing this, it is important to follow following rules:
1. Drawing up a pedigree begins with the proband. Siblings are listed in birth order from left to right, starting with the eldest.
2. All members of the pedigree are arranged strictly by generations in one row.
3. Generations are indicated by Roman numerals to the left of the pedigree from top to bottom.
4. Arabic numerals number the offspring of one generation (one row) from left to right.
5. Due to the fact that some diseases manifest themselves at different periods of life, the age of family members is indicated.
6. Personally examined members of the pedigree are noted.
The graphic representation of the pedigree can be vertical-horizontal or arranged in a circle (in the case of extensive data). The pedigree scheme is accompanied by a description of the designations under the figure, which is called a legend (Fig. 2.2).
Genetic analysis of the pedigree
The task of genetic analysis is to establish the hereditary nature of the disease and the type of inheritance, to identify heterozygous carriers of the mutant gene, as well as to predict the birth of sick children in families with hereditary pathology.
Pedigree analysis includes the following steps: 1. Establishing whether a given trait or disease is single in the family or there are several cases (family character). If a trait occurs several times in different generations, then it can be assumed that this trait has a hereditary nature. 2. Determining the type of trait inheritance. To do this, analyze the pedigree, taking into account the following points:
1) whether the studied trait is found in all generations and how many members of the pedigree have it;
2) whether its frequency is the same in both sexes and in which sex it occurs more often;
3) to persons of what sex the trait is transmitted from a sick father and a sick mother;
4) whether there are families in the pedigree in which sick children were born to both healthy parents, or healthy children were born to both sick parents;
5) what part of the offspring has an inherited trait in families where one of the parents is sick.
Autosomal dominant inheritance is characterized by the fact that the mutant gene is associated with an autosome and manifests itself in both homozygous (AL) and heterozygous (Aa) states. Because of this, the following features of inheritance can be traced:
1) transmission of pathology from sick parents to children;
2) both sexes are affected in equal proportions;
3) healthy family members usually have healthy offspring;
4) father and mother equally pass the mutant gene to daughters and sons. The disease can be passed from father to son.
The clinical manifestations of the disease can vary significantly depending on the expressivity and penetrance of the gene. Expressivity is the degree of gene expression (in our case, the severity of the disease). With a high expression of the gene, a severe, often fatal form of the disease develops, with a low expression, the person is outwardly healthy. Penetrance refers to the frequency of manifestation of a mutant gene among its carriers. It is determined by the ratio of the number of individuals with a given disease (or trait) to the number of individuals with a given gene, expressed as a percentage. For example, the penetrance of atherosclerosis is 40%, Marfan's syndrome is 30%, retinoblastoma is 80%, etc.
Depending on the type of inheritance, the overall picture of the pedigree looks different.
With an autosomal recessive type of inheritance, the mutated gene manifests its effect only in the homozygous state. For this reason, in the heterozygous state, it can exist for many generations without manifesting itself phenotypically.
With this type of inheritance, the disease is rare in the pedigree and not in all generations. The probability of the disease in girls and boys is the same. The trait can manifest itself in children whose parents were healthy, but were heterozygous carriers of the mutant gene. There are several options for such marriages:
1) mother aa x father aa - such parents will have all children sick (aa);
2) mother Aa x father aa - 50% of the children will be sick (genotype aa) and 50% phenotypically healthy (genotype Aa), but will be heterozygous carriers of the defective gene;
3) mother Aa x father Aa - 25% of children will be sick (genotype aa), 75% phenotypically healthy (genotypes AA and Aa), but 50% of them will be carriers of the mutant gene (genotype Aa).
Expressivity and penetrance vary widely (from 0 to 100%) and strongly depend on environmental conditions. According to the autosomal dominant type, polydactyly (six-fingered), brachydactyly (short-fingered), achondroplasia (dwarfism), Marfan's syndrome ("spider fingers") and other diseases are inherited (Fig. 2.3).
With a dominant type of inheritance, if one of the parents is sick (Aa), the probability of having a sick child is 50%, provided that the gene is completely penetrant. In the case of heterozygosity of both parents (Aa x Aa), sick children can be born with a probability of 75%. Many autosomal dominant diseases in the homozygous state are more severe than in heterozygotes. However, in practice it is not uncommon for carriers of a dominant gene to remain phenotypically healthy. As a result, the type of pedigree changes and gaps in generations appear.
Carrying a dominant gene without a phenotypic manifestation can be suspected in one of the parents if patients with the same dominant pathology appear among his descendants. When healthy parents have a sick child and there are other cases of this disease in the pedigree, it is legitimate to assume that one of the parents of the patient had a defective gene that did not penetrate, but was passed on to the descendant.
The dominant gene may have varying degrees of expressivity, which makes it difficult to establish an autosomal dominant mode of inheritance. Consider this on the example of a hereditary pathology of the connective tissue - Marfan's syndrome.
It is known that the incidence of hereditary recessive autosomal diseases is directly dependent on the prevalence of the mutant gene among the population. The frequency of such diseases is especially increased in isolates and among populations with a high percentage of consanguineous marriages. Such marriages have a negative effect on offspring, which is indicated by the fact that mental retardation among children from related marriages is 4 times higher than in families with unrelated marriages.
With an autosomal recessive type of inheritance (as with an autosomal dominant one), various degrees of expressiveness and penetrance of the trait are possible. Diseases with an autosomal recessive type of inheritance include many metabolic diseases, including phenylketonuria, galactosemia, albinism (Fig. 2.4), cystic fibrosis, etc. It has been established that recessive diseases are more often diagnosed at an early age.
The inheritance of sex-linked diseases is determined by the fact that the mutant gene is located on the X or Y chromosome. It is known that women have two X-sex chromosomes, and men have one X- and one Y-chromosome. In humans, more than 200 genes are located on the X chromosome. Genes located on the X chromosome can be recessive or dominant.
In women, the mutant gene may be on both X chromosomes or only one of them; in the first case it is homozygous, in the second it is heterozygous. Men, being hemizygous (have only one X chromosome), pass it only to daughters and never to sons. Any gene, both dominant and recessive, localized on its X chromosome will definitely show up. In that main feature X-linked inheritance.
X-linked recessive inheritance is characterized by the following features:
1) the disease is more common in males;
2) sick children can be born from healthy parents (if the mother is heterozygous for the mutant gene);
3) sick men do not transmit the disease to their sons, but their daughters become heterozygous carriers of the disease;
4) sick women can be born only in families where the father is sick and the mother is heterozygous for the mutant gene.
Let us consider several examples when a recessive gene is localized on the X chromosome. If a healthy woman and a sick man marry, then in such a family all children will be healthy, and daughters will receive one X chromosome with a mutant gene from their father and will be heterozygous carriers (because they will receive a second normal X chromosome from their mother) . In the event that a healthy man and a woman with a pathological gene enter into marriage, then the probability of having a sick boy will be 50% of all boys and 25% of all children.
The probability of the birth of sick girls is very low and is possible only if the father is sick and the mother is heterozygous for the mutant gene. In such a family, half of the boys will be sick. Among girls, half will show the disease, and the other half will carry the defective gene.
A classic example of recessive, sex-linked inheritance is hemophilia. Patients suffer from increased bleeding. The reason is the insufficient content of blood clotting factors in the blood. On fig. 2.5 shows the pedigree of a family with hemophilia
Pedigree analysis shows that only boys are affected. (II - 1.4; III - 7.15). This suggests that the hemophilia gene is sex-linked. Sick children are more often born from healthy parents and, therefore, the disease gene is recessive.
It is known that hemophilia is widespread in the royal families of Europe. This is due to the conclusion of closely related marriages. As a result, the resulting mutations persisted within the family. Queen Victoria of England was a carrier of the hemophilia gene. Her son Leopold was born a hemophiliac. Through her daughters and grandchildren, Queen Victoria passed on the hemophilia gene to Voldemar and Henry of Prussia, Friedrich of Hesse, Tsarevich Alexei Romanov, Ruprecht of Tech-Athlon, two Battenberg and two Spanish princes (Figure 2.6). In addition to hemophilia, recessive genes are localized on the X chromosome, causing Duchenne myopathy, some forms of color blindness, and other diseases.
When the dominant gene is localized on the X chromosome, the type of inheritance is called X-linked dominant. It is characterized by the following features:
1) both men and women are sick, but there are twice as many sick women as sick men;
2) the disease can be traced in every generation;
3) if a father is sick, then all his daughters will be sick, and all sons will be healthy;
4) if the mother is sick, then the probability of having a sick child is 50%, regardless of gender;
5) children will be sick only if one of the parents is sick;
6) healthy parents will have all children healthy.
According to the X-linked dominant type, phosphatemia (lack of phosphate in the blood), brown color of tooth enamel, etc. are inherited.
It has its own characteristics and Y-linked inheritance.
Few genes are located on the Y chromosome in males. They are only passed on to sons and never to daughters (Holandric inheritance). With the Y chromosome in men, traits such as hypertrichosis (presence of hair along the edge of the auricles), skin membranes between the toes, development of the testes, growth rate of the body, limbs and teeth are inherited. Characteristic features of inheritance with the Y-chromosome can be seen in Fig. 2.7.